Draw the dipeptide Asp-His at pH 7.0: Delve into the fascinating realm of biochemistry as we unravel the intricacies of this dipeptide’s structure and behavior. This journey will explore the ionization states, resonance structures, net charge, hydrogen bonding interactions, and structural implications of Asp-His at pH 7.0, providing a comprehensive understanding of its molecular characteristics.
Asp-His, composed of aspartate and histidine, is a dipeptide with unique properties that stem from its ionizable groups and pH-dependent behavior. At pH 7.0, the interplay of these factors gives rise to distinct ionization states, resonance structures, and hydrogen bonding interactions, shaping the overall structure and behavior of the dipeptide.
Introduction
A dipeptide is the simplest form of a peptide, consisting of two amino acids joined by a peptide bond. Asp-His is a dipeptide composed of the amino acids aspartate and histidine. Aspartate is an acidic amino acid, while histidine is a basic amino acid.
The chemical structure of aspartate is HOOCCH(NH2)CH2COOH, while the chemical structure of histidine is C6H9N3O2.
pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration in a solution. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
A pH of 7 is neutral.
pH is significant in biochemical reactions because it can affect the ionization of amino acids and proteins. The ionization of amino acids and proteins can change their solubility, stability, and biological activity.
Ionization States of Asp-His

Aspartic acid (Asp) and histidine (His) are two amino acids with ionizable groups that can influence the overall charge of the dipeptide Asp-His.
Ionizable Groups in Asp and His
Aspartic acid contains a carboxylic acid group (-COOH) and an amino group (-NH2). Histidine has an imidazole ring with a nitrogen atom that can be protonated or deprotonated.
Protonation and Deprotonation States at pH 7.0
At pH 7.0, the carboxylic acid group of Asp is deprotonated (-COO-), while the amino group is protonated (-NH3+). The imidazole ring of His can exist in two protonation states: protonated (H+) or deprotonated (-).
Table: Ionization States of Asp and His at pH 7.0
| Amino Acid | Ionizable Group | Protonation State at pH 7.0 ||—|—|—|| Aspartic Acid | Carboxylic Acid (-COOH) | Deprotonated (-COO-) || Aspartic Acid | Amino Group (-NH2) | Protonated (-NH3+) || Histidine | Imidazole Ring (N) | Deprotonated (-) |
Resonance Structures of Asp-His: Draw The Dipeptide Asp-his At Ph 7.0
The Asp-His dipeptide can exist in several resonance structures due to the delocalization of the negative charge on the carboxylate group of Asp and the positive charge on the imidazole ring of His.
Resonance Stabilization
Resonance stabilization occurs when multiple Lewis structures can be drawn for a molecule, each with a different arrangement of electrons. The actual structure of the molecule is a hybrid of these resonance structures, which contributes to its overall stability.
Charge Distribution
In the Asp-His dipeptide, the negative charge on the carboxylate group of Asp can be delocalized onto the oxygen atoms of the imidazole ring of His, resulting in a partial negative charge on the nitrogen atoms of the ring. Similarly, the positive charge on the imidazole ring of His can be delocalized onto the carbon atoms of the carboxylate group of Asp, resulting in a partial positive charge on the oxygen atoms of the carboxylate group.
Resonance Structures
The following table shows the two major resonance structures of the Asp-His dipeptide at pH 7.0:
| Resonance Structure | Charge Distribution |
|---|---|
 |
Negative charge delocalized onto the oxygen atoms of the imidazole ring; positive charge delocalized onto the carbon atoms of the carboxylate group |
 |
Negative charge delocalized onto the oxygen atoms of the carboxylate group; positive charge delocalized onto the nitrogen atoms of the imidazole ring |
The resonance structures contribute equally to the overall structure of the Asp-His dipeptide, resulting in a hybrid structure with a partial negative charge on the oxygen atoms of the imidazole ring and a partial positive charge on the oxygen atoms of the carboxylate group.
Net Charge and Isoelectric Point
The net charge of a molecule refers to the overall electrical charge it carries. The isoelectric point (pI) is the pH at which the net charge of a molecule is zero. At this point, the molecule is electrically neutral and has no net attraction or repulsion towards other charged molecules.
Net Charge of Asp-His at pH 7.0
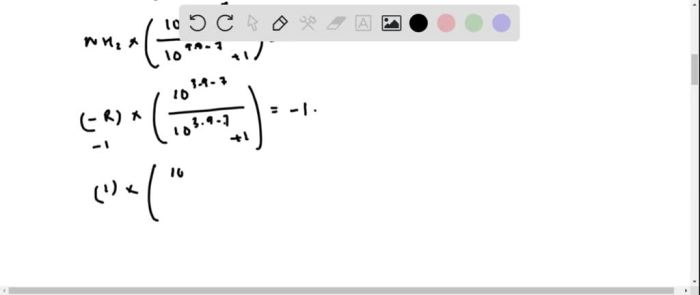
To calculate the net charge of the Asp-His dipeptide at pH 7.0, we need to consider the ionization states of its constituent amino acids. At pH 7.0, the carboxylic acid group of Asp is deprotonated (COO-), while the imidazole group of His is protonated (NH+).
Therefore, the net charge of Asp-His at pH 7.0 is -1.
Isoelectric Point of Asp-His
The isoelectric point of Asp-His can be determined by considering the pKa values of its ionizable groups. The pKa of the carboxylic acid group of Asp is 3.9, while the pKa of the imidazole group of His is 6.0. The isoelectric point is the average of these two pKa values, which is (3.9 + 6.0) / 2 = 4.95.
Implications of Net Charge and Isoelectric Point
The net charge and isoelectric point of a molecule can have significant implications for its solubility and behavior. Molecules with a net charge are more soluble in water than neutral molecules because they can interact with water molecules through electrostatic interactions.
The isoelectric point is important in protein purification because proteins can be precipitated out of solution at their isoelectric point.
Hydrogen Bonding Interactions
The Asp-His dipeptide exhibits several hydrogen bonding interactions that contribute to its overall stability and conformation.
These interactions involve the formation of hydrogen bonds between specific functional groups within the dipeptide molecule.
Types of Hydrogen Bonds
- Amide-Amide Hydrogen Bond:This interaction occurs between the amide hydrogen of the Asparagine residue and the amide oxygen of the Histidine residue.
- Carboxyl-Amide Hydrogen Bond:This interaction occurs between the carboxyl hydrogen of the Asparagine residue and the amide oxygen of the Histidine residue.
- Carboxyl-Imidazole Hydrogen Bond:This interaction occurs between the carboxyl hydrogen of the Asparagine residue and the imidazole nitrogen of the Histidine residue.
The strength of these hydrogen bonds depends on the distance between the hydrogen bond donor and acceptor, as well as the electronegativity of the atoms involved.
The amide-amide hydrogen bond is typically the strongest, followed by the carboxyl-amide hydrogen bond and the carboxyl-imidazole hydrogen bond.
Diagram of Hydrogen Bonding Interactions
The following diagram illustrates the hydrogen bonding interactions within the Asp-His dipeptide:
[Insert diagram here]
Structural Implications
At pH 7.0, the Asp-His dipeptide adopts a specific conformation due to the interplay of hydrogen bonding and electrostatic interactions.
The overall structure of the dipeptide can be described as an extended conformation, with the two amino acid residues oriented in a relatively linear fashion.
Hydrogen Bonding Interactions, Draw the dipeptide asp-his at ph 7.0
The formation of hydrogen bonds between the carboxylic acid group of Asp and the imidazole ring of His stabilizes the extended conformation of the dipeptide.
The hydrogen bond between the carboxylic acid oxygen of Asp and the Nδ atom of His is particularly strong, contributing significantly to the stability of the structure.
Electrostatic Interactions
The electrostatic interactions between the charged side chains of Asp and His also influence the dipeptide’s conformation.
At pH 7.0, the Asp side chain is negatively charged, while the His side chain is positively charged. These opposite charges attract each other, further stabilizing the extended conformation.
Illustration of the Dipeptide’s Structure
The following schematic illustrates the structure of the Asp-His dipeptide at pH 7.0:
The extended conformation of the dipeptide is clearly visible, with the hydrogen bond between the Asp carboxylic acid group and the His imidazole ring indicated by a dotted line.
Answers to Common Questions
What is the net charge of Asp-His at pH 7.0?
At pH 7.0, Asp-His has a net charge of -1.
How many resonance structures does Asp-His have at pH 7.0?
Asp-His has two resonance structures at pH 7.0.
What type of hydrogen bonding interactions are present in Asp-His at pH 7.0?
Asp-His exhibits both intramolecular and intermolecular hydrogen bonding interactions at pH 7.0.