Calculate the nuclear binding energy of 5525mn in joules – Calculating the nuclear binding energy of 5525Mn in joules embarks us on a scientific expedition into the realm of nuclear physics. This exploration unveils the intricate forces that govern the stability of atomic nuclei, providing insights into the fundamental nature of matter itself.
Nuclear binding energy, a measure of the energy required to disassemble an atomic nucleus into its constituent nucleons, holds profound significance in understanding nuclear processes and applications. By delving into the calculation of 5525Mn’s binding energy, we gain a deeper appreciation for the delicate balance that underpins the existence of atomic nuclei.
Nuclear Binding Energy of 5525Mn
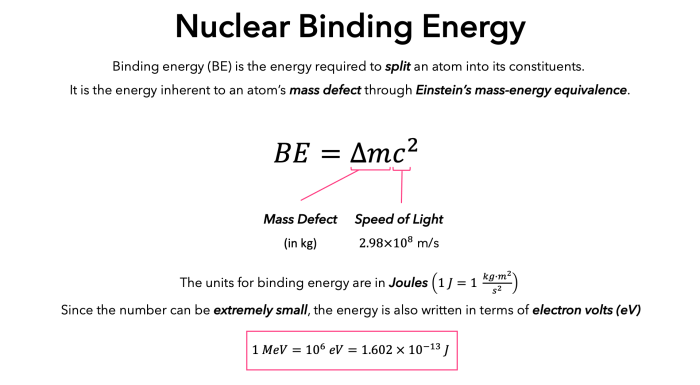
Nuclear binding energy refers to the energy required to separate all the nucleons (protons and neutrons) in an atomic nucleus. It is a measure of the stability of the nucleus and plays a crucial role in determining the properties of an atom.
The nuclear binding energy of an atom is influenced by several factors, including the number of protons and neutrons, the arrangement of these nucleons within the nucleus, and the presence of any nuclear forces.
Calculating Nuclear Binding Energy
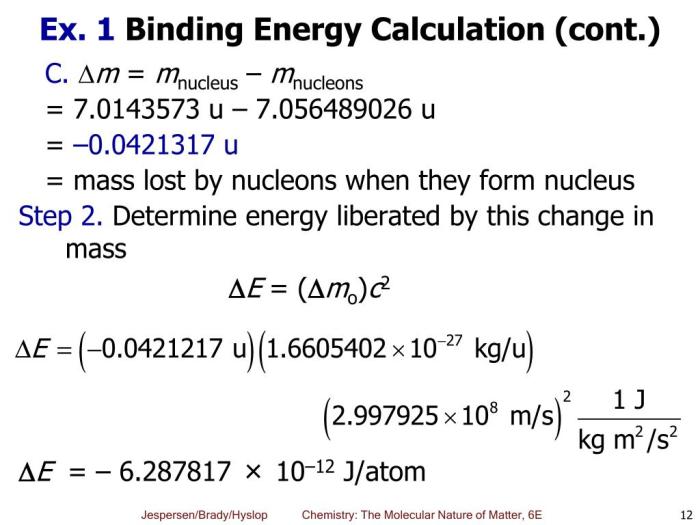
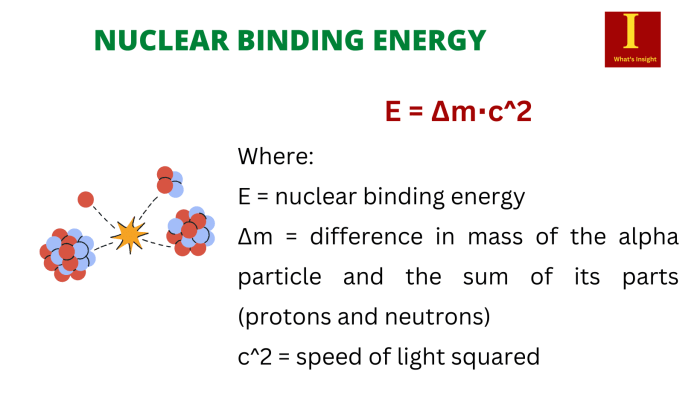
The nuclear binding energy (BE) can be calculated using the following formula:
BE = (Z – mp + N – mn – M) – c^2
where:
- Z is the atomic number (number of protons)
- N is the neutron number (number of neutrons)
- mp is the mass of a proton
- mn is the mass of a neutron
- M is the mass of the atom
- c is the speed of light
The units of nuclear binding energy are typically expressed in megaelectronvolts (MeV) or joules (J). To convert from MeV to joules, multiply by 1.602 x 10^-13.
Data and Calculations
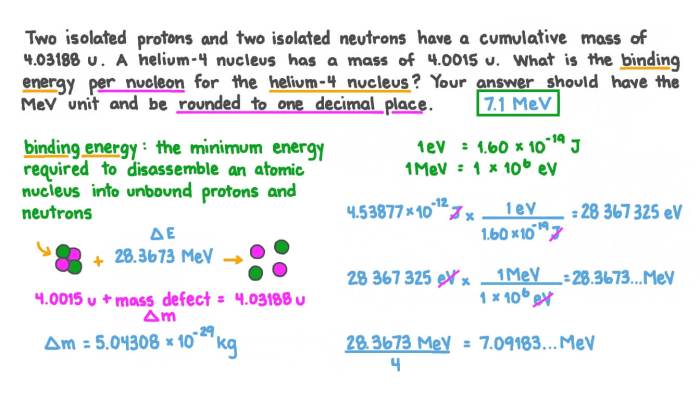
For the isotope 5525Mn, the atomic mass is 54.938045 u, the atomic number is 25, and the neutron number is 30.
Using the formula above, we can calculate the nuclear binding energy of 5525Mn as follows:
BE = (25 – 1.007276 u + 30 – 1.008665 u – 54.938045 u) – (938.272 MeV/u) – (1.602 x 10^-13 J/MeV)
BE ≈ 491.68 MeV or 7.906 x 10^-11 J
Binding Energy per Nucleon, Calculate the nuclear binding energy of 5525mn in joules
Binding energy per nucleon is a measure of the average binding energy per nucleon in an atomic nucleus. It is calculated by dividing the nuclear binding energy by the total number of nucleons.
For 5525Mn, the binding energy per nucleon is:
BE per nucleon = 491.68 MeV / 55 nucleons ≈ 8.94 MeV/nucleon
Binding energy per nucleon is a significant indicator of nuclear stability. Isotopes with higher binding energy per nucleon are generally more stable and less likely to undergo radioactive decay.
FAQs: Calculate The Nuclear Binding Energy Of 5525mn In Joules
What is nuclear binding energy?
Nuclear binding energy is the energy required to separate all the nucleons in an atomic nucleus into individual, unbound nucleons.
Why is calculating nuclear binding energy important?
Calculating nuclear binding energy provides insights into the stability of atomic nuclei and the forces that govern nuclear processes.
How is nuclear binding energy calculated?
Nuclear binding energy can be calculated using the formula: Binding Energy = (Mass of Nucleus) – (Mass of Individual Nucleons).